Abstract
Introduction:
Targeted ABL kinase inhibitors (TKIs) have shown great activity in Ph+ Acute Lymphoblastic Leukemia (Ph+ ALL), however relapsed disease remains an unmet need. The bispecific antibody blinatumomab was recently approved as a single agent for use in patients with Ph+ ALL and there is much interest in combining this with targeted therapies. Second generation ABL kinase inhibitors inhibit both Src and LYN in addition to ABL. This is of particular interest in Ph+ ALL as LYN is important for leukemogenesis. T cell receptor (TCR) signaling is also dependent upon Src family kinase activity, and Src inhibitors may impact the efficacy of immunotherapies reliant on native T cell function. We sought to investigate the in vitro effects of ABL specific vs dual Src/ABL kinases on blinatumomab efficacy in both healthy donor as well as primary patient samples.
Methods:
We isolated peripheral blood mononuclear cells (PBMC) via Ficoll-Hypaque gradient from five healthy donors as well as from two patients with de novo and one patient with relapsed Ph+ ALL who harbored a T315I mutation. PBMC were labeled with CellTrace Violet and cultured for 5 days with no stimulation, blinatumomab, or blinatumomab in combination with imatinib, dasatinib, ponatinib or nilotinib at varying concentrations. Immunophenotyping was performed using multi-parameter flow cytometry for the following cell surface markers: CD45, CD3, CD4, CD8, CD56, and CD19. Blinatumomab efficacy was assessed by comparing the numbers of CD19+ / CD3- cells in untreated samples to those that had been treated with blinatumomab in the presence or absence of TKIs. Cell division of T cells was measured by CellTrace Violet dilution. Cytokine production was assessed via LEGENDplex Human Th Cytokine Panel. Levels of total Src, phospho-Src, total LCK and phospho-LCK were assessed via immunoblot.
Results:
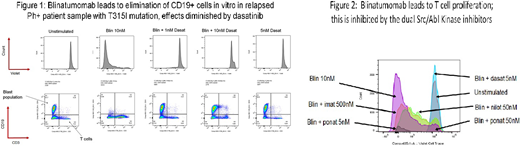
After 5 days of exposure, blinatumomab led to T-cell proliferation in both healthy donor and patient PBMCs. Proliferation was observed in both CD8+ and CD4+ T cell subsets, although the effect was more pronounced in CD8+ cells. T cell proliferation, however, was completely suppressed by either dasatinib or ponatinib at nanomolar concentrations. This effect was far less pronounced with the ABL kinase inhibitors imatinib and nilotinib. Treatment of PBMCs with blinatumomab led to increased production of the cytokines IFN-g, IL-17-a and IL-22 in patient samples and healthy donors, while levels of IL-6 were increased in the patient samples only and levels of IL-10 in healthy subjects only. Cytokine production was absent in samples treated with blinatumomab and either dasatinib or ponatinib, while levels of IFN-g, IL-17a and IL-22 were minimally affected when blinatumomab was combined with imatinib. Immunoblots confirmed that dasatinib and ponatinib but not imatinib nor nilotinib inhibited phosphorylation of total Src as well as of LCK, likely explaining the inhibitory effects of these agents. In patient samples, blinatumomab alone and the TKIs alone greatly reduced the number of CD19+ cells. However, when dasatinib and blinatumomab were combined in the sample with a T315I mutation, there was little reduction in the percentage of CD19+ cells and no amplification of CD3+ cells, suggesting that dasatinib was able to inhibit the cytotoxic effects of blinatumomab with no effect to the leukemic cells.
Discussion:
Our results suggest that the combination of dual Src/ABL inhibitors with blinatumomab may abrogate the effects of blinatumomab by directly inhibiting T cell function. This is likely via inhibition of LCK, a known member of the TCR signaling pathway. Although small case series have reported responses in patients treated with blinatumomab and TKIs, it is possible that the majority of the response is from the TKI rather than blinatumomab. Only a randomized trial of a TKI +/- blinatumomab would be able to discern whether there is benefit of adding a dual Src/ABL TKI to bispecific antibody therapy. While our data are limited by sample numbers and by the fact that responses in living subjects may differ according to many other complex interactions in the in vivo immune microenvironment, the potential immunomodulatory effects of targeted therapies should be taken into consideration before they are combined with immunotherapies.
Leonard:Amgen: Research Funding. Druker:McGraw Hill: Patents & Royalties; Fred Hutchinson Cancer Research Center: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; ARIAD: Research Funding; Monojul: Consultancy; Millipore: Patents & Royalties; Novartis Pharmaceuticals: Research Funding; Oregon Health & Science University: Patents & Royalties; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Meyers Squibb: Research Funding; ALLCRON: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beta Cat: Membership on an entity's Board of Directors or advisory committees; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Patient True Talk: Consultancy; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; Third Coast Therapeutics: Membership on an entity's Board of Directors or advisory committees; GRAIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Consultancy; Henry Stewart Talks: Patents & Royalties; Aptose Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Tyner:Constellation: Research Funding; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Gilead: Research Funding; Aptose: Research Funding; Incyte: Research Funding; Genentech: Research Funding; Array: Research Funding; Takeda: Research Funding; AstraZeneca: Research Funding. Lind:Celgene: Research Funding; Janssen Pharmaceutical R&D: Research Funding; Amgen: Research Funding; Fluidigm: Honoraria; Monojul: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal